On May 25th, 2020, a research article entitled Nickel-Catalyzed Allylmethylation of Alkynes with Allylic Alcohols and AlMe3: Facile Access to Skipped Dienes and Trienes was accepted to the internationally prestigious journal Angewandante Chemie Internal Edition (Angew. Chem. Int. Ed. 2020, 59, 10.1002/anie.202006322). Assoc. Prof. Dr. Wanfang Li from the College of Science, USST, is the first and co-corresponding author of this high impact paper. This research work on MCR alkyne dicarbofunctionalization was carried out with an international cooperation with Prof. Yu Zhao, the head of Department of Chemistry, National University of Singapore.
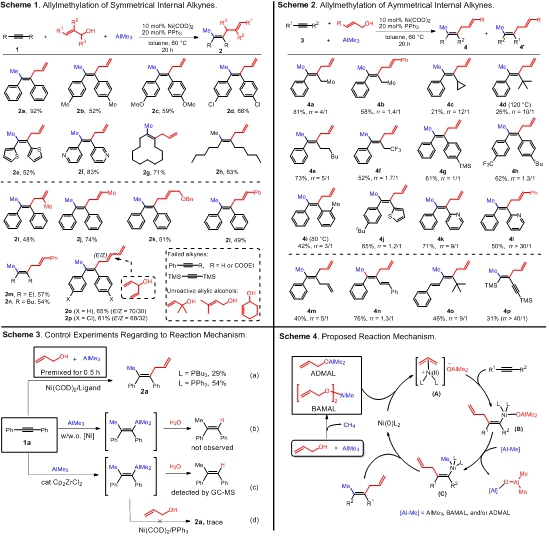
All-carbon tetrasubstituted alkenes exist as a core structure in numerous bioactive compounds, functional materials, and also serve as versatile intermediates in chemical synthesis. For the synthesis of such compounds, transition-metal-catalyzed difunctionalization of alkynes, and specifically the addition of one carbon nucleophile and one carbon electrophile across the internal alkynes, represents a highly efficient strategy. Many effective stepwise functionalization procedures have been documented.This simple catalytic procedure utilizes commercially available Ni(COD)2, triphenylphosphine, and inexpensive reagents, and delivers valuable skipped dienes and trienes with an all-carbon tetrasubstituted alkene unit in a highly stereoselective fashion. Both symmetric (Scheme 1) and asymmetric (Scheme 2) internal alkynes are applicable substrates for this useful transformation. Preliminary mechanistic studies (Scheme 3) support the reaction pathway of allylnickelation followed by transmetalation in this dicarbofunctionalization of alkynes (Scheme 4).